Resonance Structure No3
The negative charge on this ion is delocalized due to resonance. The nitrate anion has a trigonal planar structure in which 3 oxygen atoms are bonded to a central nitrogen atom.

No3 Resonance Structures Nitrite Ion
Unit 3 Atomic Structure.

. Discovery of subatomic particles electron proton and neutron. Lewis picture reverberation structures VSEPR model molecular shapes Covalent Bond. While discussing compounds of nitrogen with a friend.
H2 SNO3 NO2 S8 acidic Balance all atoms other than H and O. Cell envelope cell membrane cell wall. The structure of a NaNO 3 molecule is illustrated below.
Pulsed Multifrequency Electron Paramagnetic Resonance Spectroscopy Reveals Key Branch Points for One- vs Two-Electron Reactivity in MnFe Proteins. The ion is the conjugate base of nitric acid consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. I in a rather a carefree tone mentioned that the fourth bond of nitrogen is always a coordinate bond because a covalent bond would require equal sharing of electrons and that would leave nitrogen with an extra electron making a total of 9 electrons and thus break the octet rule which holds valid for the nitrogen.
The nitrate ion carries a formal charge of 1. Shafaat Journal of the American Chemical Society 2022 144 27 11991-12006 Article Publication Date Web. Molecular orbital theory- Methodology Orbital energy level chart Bond request Magnetic properties for homonuclear diatomic species.
Endomembrane system-endoplasmic reticulum Golgi bodies lysosomes vacuoles. Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. For analytical study of the prepared sample the amount of absorption within wave length of 300550 nm was observed by uv-vis spectroscopy.
It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a. Sodium nitrate features an ionic bond between one Na ion and one NO 3 ion. Atoms can be stable even though the number of valence electrons in the atoms in a molecule is more.
Mitochondria ribosomes plastids micro bodies. Plant cell and animal cell. This charge results from a combination formal charge in which each of the three oxygens carries a 2 3 charge whereas the nitrogen carries a 1 charge all these.
Cell organelles-structure and function. Cell theory and cell as the basic Unit of life. Cell Structure and Function.
This product is 100 funded by the MoSTEMWINs 197 million grant from the US. Noncovalent H-Bonding Interactions Molecular Orbital Analysis Thermodynamics and Lowest. The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
Atoms can be stable even though the number of valence electrons in the atoms in a molecule is less than 8 and is called incomplete octet. 8H2 SNO3 NO2 S8 acidic The o. MD-DFT Computational Studies on the Mechanistic and Conformational Parameters for the Chemoselective Tyrosine Residue Reactions of G-Protein-Coupled Receptor Peptides with CpRhH 2 O 3OTf 2 in Water To Form Their η 6-CpRh-Tyr -GPCR peptide 2 Complexes.
It is known that an absorption band at about 370 nm due to surface plasmon resonance in ZnO nanoparticles. Using the electronegativity values in Figure PageIndex1 arrange the following covalent bondsall commonly found in amino acidsin order of increasing polarity. Valence Bond Theory- Orbital overlap Directionality of bonds.
For information on South Africas response to COVID-19 please visit the COVID-19 Corona Virus South African Resource Portal. Structure of prokaryotic and eukaryotic cell. Then designate the positive and negative atoms using the symbols δ and δ.
If the temperature of 1280 grams of ethanol increases from 260C to 740 C how much heat has bee. 1 shows the UV-Vis spectra of ZnO nanoparticles recorded between 300 and 550 nm. Thomson and Rutherford atomic models and their limitations.
Nature of electromagnetic radiation Photoelectric effect. Kisgeropoulos Yunqiao J. Bond polarities play an important role in determining the structure of proteins.
The spectrum of the hydrogen atom Bohr model of hydrogen atom its postulates derivation of the relations for the energy of the electron and radii of the. Department of Labor Employment and Training Administration TAACCCT.
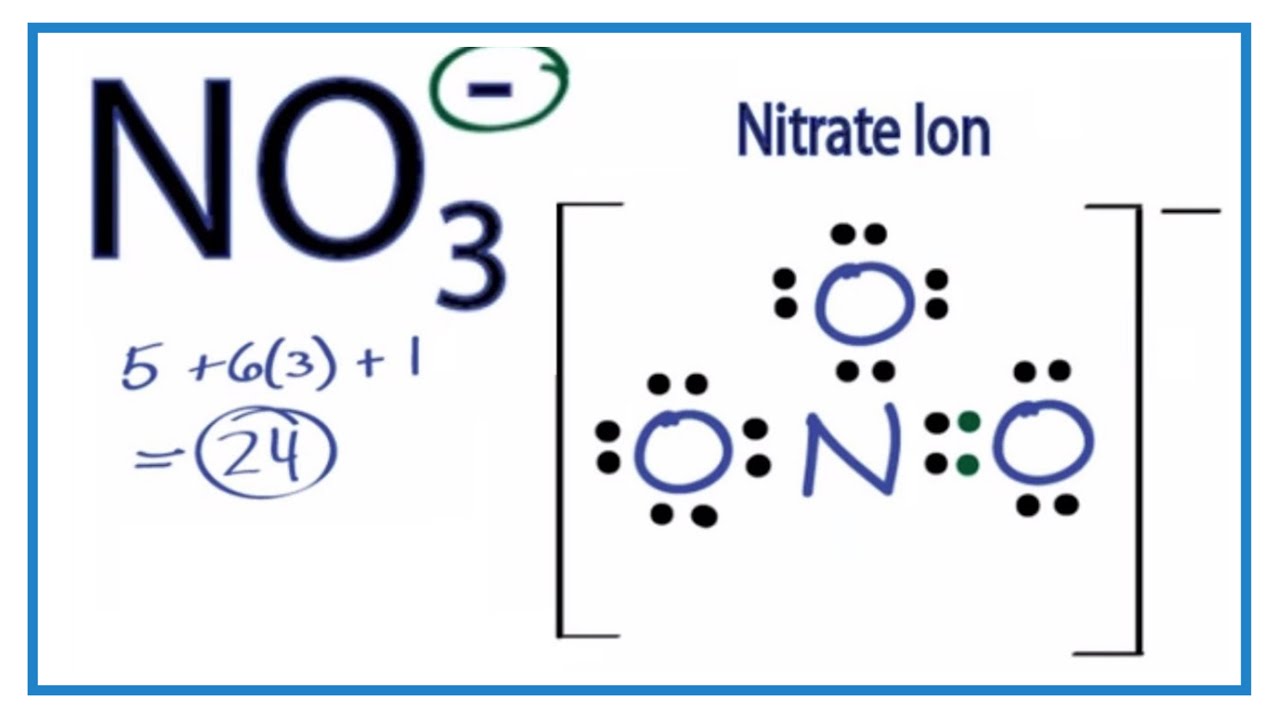
No3 Lewis Structure How To Draw The Lewis Structure For No3 Youtube

Resonance Structures For No3 Nitrate Ion Youtube
Resonance Structures And Calculated Mulliken Charges Of No 3 A Download Scientific Diagram

How Would You Draw All The Resonance Structures For Nitrate No3
No comments for "Resonance Structure No3"
Post a Comment